New Breakthrough In Battery Tech Offers Prospect of 3-4 Times Battery Capacity
 Mobile devices have always been hampered by one thing more than any other: battery life. To have that small, slim iPhone that feels thin and light in your hand, the battery has to be kept small too. The 1560 mAh capacity of the iPhone 5S pales in significance to other “phablets” often possessing a 3,000 mAh battery, but it does ensure that the Apple “wonderphone” stays svelte.
Mobile devices have always been hampered by one thing more than any other: battery life. To have that small, slim iPhone that feels thin and light in your hand, the battery has to be kept small too. The 1560 mAh capacity of the iPhone 5S pales in significance to other “phablets” often possessing a 3,000 mAh battery, but it does ensure that the Apple “wonderphone” stays svelte.
The challenge for scientists has been to make lithium batteries more efficient so that either the same capacity battery can deliver several times the power usage or a smaller battery can power devices as well as a larger lithium battery can do today. Now scientists at Stanford have got around teething issues with the lithium ion battery technology to solve long standing problems with how to develop a battery that will really last.
Stanford Battery Experts
Yi Cui and his team at Stanford have led the way with battery breakthroughs and this is no exception. The techo-babble is a little difficult to digest, but bear with us...
The current lithium ion battery has a certain amount of energy output until it runs out of power. This is essentially controlled by how many lithium ions can go into the anode during the charging process. The more that are present, the greater the stored charge for use the next day.
Lithium Volatility In The Mix
Graphite is actually a cheaper, better option than lithium because it holds capacity much longer but it is heavy with a useful energy of 305 mAh per gram. Lithium battery charge dissipates much faster but it can store 3,860 mAh per gram. The difficulty with lithium has been holding the charge and the volatile nature of the material as it expands during the charging process cracking the metal covering.
Lithium anodes develop dangerous cracks and dendrites are formed which creates the volatility issue. The dendrites are metal deposits that form when lithium ions leak out from the battery to the metal casing. This process gradually lowers the battery charging efficiency and creates the combustion problem.
New Approach Is Needed
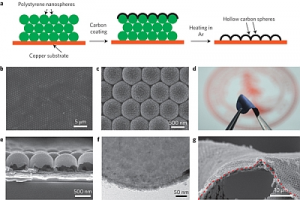
The team at Stanford led by Yi Cui has now found a way to generate lithium anodes that can hold their capacity over more than 150 cycles of charging up and discharging during use whilst keeping the lithium non-volatile so that it won't explode. Thanks to nano technology, Stanford's carbon nanosphere coating gets around these tricky issues by layering a series of dome-like carbon nanospheres just 20 nanometers thick on the lithium anode surface which prevents the leakage and maintains the lithium efficiency in the process.
Battery Efficiency Levels
In lab testing, the lithium anode maintains 99% efficiency after 150 charge/discharge cycles have been completed. This is not yet at the 99.9% level generally required for commercial use but Cui is confident that with more time and some clever re-engineering they can improve their results still further.
At that point, a new breed of rechargeable batteries could hit the market that will deliver 3-4 times the efficiency than before. Good news for users who find their smartphone battery doesn't give them much more than a 4-6 hour charge before it is badly in need of a wall charging socket or a power bank to add some more juice.
It is probably still a handful of years away from a commercial version that we'll all be able to benefit from that in the future. We'll have the smart guys working with Mr Cui to thank for it when we do.
Research Paper
For anyone interesting in the technology behind these new batteries, Cui and other researchers have jointly published a paper in Nature Nanotechnology entitled: "Interconnected hollow carbon nanospheres for stable lithium metal anodes."
